Open Access, Volume 9
Optimizing atrioventricular synchrony in young adults implanted with leadless pacemakers
Strik M1,2*; Ploux S1,2; Ramirez FD3,4; Racine HP1; Clementy N5; Mondoly P6; Eschalier R7; Haïssaguerre M1,2; Bordachar P1,2
1Bordeaux University Hospital (CHU), Cardio-Thoracic Unit, F-33600 Pessac, France.
2IHU Liryc, Electrophysiology and Heart Modeling Institute, fondation Bordeaux Université, F-33600 Pessac- Bordeaux, France.
3Division of Cardiology, University of Ottawa Heart Institute, Ottawa, Ontario, Canada.
4School of Epidemiology and Public Health, University of Ottawa, Ottawa, Ontario, Canada.
5Cardiology Department, University Hospital Trousseau, Tours, France.
6Department of cardiology, University Hospital Rangueil, Toulouse, France.
7Cardiology Department, University Hospital Clermont-Ferrand, Clermont-Ferrand, France.
Dr Strik M
Service Pr Haïssaguerre, Hôpital cardiologique du Haut-Lévêque, Avenue de Magellan, 33600 Pessac,
France.
Tel: +33 57656471; Email: marcstrik@gmail.com
Received : February 18, 2023,
Accepted : March 24, 2023
Published : March 31, 2023,
Archived : www.jclinmedcasereports.com
Abstract
The MicraTM AV is a second generation single chamber leadless pacemaker which enables atrioventricular (AV) synchronization through use of its 3-axis accelerometer (previously only used for rate modulation). Although AV-synchronous pacing provides several advantages compared with ventricular-only pacing, achieving adequate AV synchrony with this system can be challenging, particularly in young active patients. We herein describe clinical cases of patients aged 40 years or younger with MicraTM AV devices to illustrate approaches to troubleshooting common issues encountered in this population and to highlight aspects of appropriate patient selection.
Keywords: Pacemaker; leadless; Micra; AV; Atrioventricular.
Copy right Statement: Content published in the journal follows Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). © Strik M(2023)
Journal: Open Journal of Clinical and Medical Case Reports is an international, open access, peer reviewed Journal mainly focused exclusively on the medical and clinical case reports.
Citation: Strik M, Ploux S, Ramirez FD, Racine HP, Clementy N, et al. Optimizing atrioventricular synchrony in young adults implanted with leadless pacemakers. Open J Clin Med Case Rep. 2023; 2002.
Introduction
Leadless pacemakers offer an alternative to conventional transvenous pacing systems, particularly for patients at an increased risk of infection or with limited venous access [1-3]. First-generation leadless pacemakers were only capable of single-chamber ventricular pacing, which significantly limited their potential indications. To widen the spectrum of patients who qualify for leadless pacing, a second generation was developed that offers atrioventricular (AV) synchronization. Using the device’s existing 3-axis accelerometer (previously only used for rate modulation) mechanical atrial contraction is sensed, which triggers ventricular pacing when programmed in VVD mode [4].
Allowing for more ‘physiological’ activation, MicraTM AV leadless pacemakers may offer benefits that are particularly appealing for young adults, including reduced physical restrictions, improved cosmesis, preservation of venous access, and elimination of the risks of pocket erosions and lead fractures [5]. However, although AV-synchronous pacing provides several advantages compared with ventricular-only pacing, achieving adequate AV synchrony with this system can be challenging. Real-world studies have shown that the percentage of AV synchronization can be modest and is dependent on patients’ activities and intrinsic AV conduction [4]. Extensive programming optimization is often required to achieve adequate AV synchrony at rest, and AV synchrony is often lost during physical activity as the device switches to VVIR mode at higher heart rates. Therefore, the programming and optimization of this system in young patients can be particularly difficult. We herein describe clinical cases of patients aged 40 years or younger with MicraTM AV devices to illustrate approaches to troubleshooting common issues encountered in this population and to highlight aspects of appropriate patient selection.
Methods
Basic principles of the MicraTM AV
The device’s built-in 3-axis accelerometer can detect intracardiac signals corresponding to the various phases of the cardiac cycle: tricuspid and mitral valve closure (A1), aortic and pulmonic valve closures (A2), passive ventricular filling (A3), and atrial contraction (A4). The A3 and A4 signals correspond to the E and A inflow waves seen on echocardiography. The A7 signal can occur at higher heart rates when the A3 and A4 signals fuse. Raw accelerometer signals are filtered and rectified by the device. Several programmable intervals can be used to troubleshoot and optimize pacemaker function. The algorithm incorporates a post-ventricular atrial blanking period – a programmable interval used to blank the A1 and A2 signals – followed by A3 and A4 sensing windows. Since A3 and A4 signals can merge, particularly at higher heart rates, the algorithm incorporates a dual threshold detection method during tachycardia: a higher A3 threshold to avoid sensing ventricular A3 signals but low enough to sense the fused A7 signal, and a lower A4 threshold to detect the A4 signal later in diastole. The end of the A3 detection window is marked as “VE” or ventricular-end period on the programmer. A4 is denoted by “AM” (atrial mechanical), typically occurring approximately 100 ms after P wave onset and starting a short programmable AV interval before ventricular pacing.
Correctly detecting the various intracardiac signals is crucial to achieving AV synchrony. A manual atrial mechanical (MAM) test is performed to line up the A1 to A4 waves under the corresponding surface ECG signals and to allow proper adjustments of the A4 threshold, A3 threshold, and A3 window, among other parameters. The MAM test can be initially run in the Ventricular Dual-Inhibited (VDI) mode with the auto atrial mechanical features turned off, to allow for clearer distinctions between the atrial signals and to prevent the device from autocorrecting and changing the programmed intervals. The MAM test can then be run in the ventricular dual response (VDD) mode to make adjustments. The auto features can be turned on to verify that the appropriate values are programmed.
MicraTM AV algorithms include
• A rate smoothing feature designed to maintain AV synchrony during intermittent A4 undersensing (programmable rate smoothed interval).
• A Conduction Mode Switch algorithm (also called “VVI+”) designed to avoid unnecessary right ventricular pacing in non-dependent patients and to promote intrinsic conduction by periodically dropping into VVI with a lower rate of 40 bpm. When the spontaneous ventricular rate is above 40 bpm the MicraTM AV works in VVI mode whereas when the intrinsic rate is below 40 bpm the device switches back to the VDD mode.
• Rate-responsive mode switching, which transitions to VDIR mode with activity to provide rate-responsive pacing.
Included patients
Seven patients (18-40 years of age) were implanted at our center with a MicraTM AV system and had follow-up of at least 3 months. The indications for pacemaker implants in this group were congenital complete heart block in three patients, post-surgery complete heart block in one patient, complete heart block in a patient with myopathy, and paroxysmal syncopal complete heart block in two patients. The protocol was approved by the ethics committee of Bordeaux University. All patients provided written and informed consent.
Implantation procedure and follow-up
The implant procedure is the same for MicraTM AV and MicraTM VR, has been already described elsewhere, and was performed by experienced implanters at our center according to standard practice. The MicraTM device was systematically placed under fluoroscopic guidance in the septal portion of the right ventricle. After implantation, the device was programmed to VDD mode and atrial sensing parameters were adjusted automatically by the device via the atrial sensing setup. Devices were interrogated prior to discharge, with MAM testing used to optimize atrial sensing features and maximize atrial tracking in sinus rhythm. Devices were interrogated three months after implantation. All patients received a smartwatch (Withings Move ECGTM) which enabled the registration of ECGs at home. Patients were instructed to register an ECG once a week and when symptomatic.
Patient 1: Implantation for paroxysmal AV block
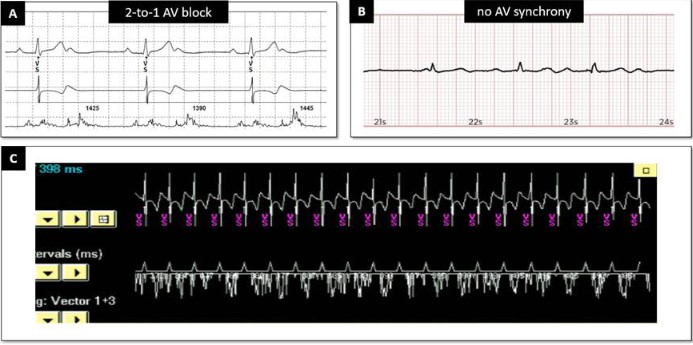
Patient 1 was a 39-year-old woman with recurrent syncope and documented paroxysmal AV block. Her echocardiogram was normal as was her baseline ECG. She was implanted with a MicraTM AV without complication. Three months after implantation she remained asymptomatic. Device interrogation at the time showed that the percentages of atrial mechanical sensed–ventricular pacing (AM-VP) and VP-only were <0.1%, with 99.9% of ventricular events being ventricular-sensed (VS) (Figure 1A), consistent with AV conduction mode switch. This algorithm, also known as VVI+ mode, is designed to promote intrinsic AV conduction and to limit right ventricular pacing in patients with intermittent AV block. Periodic conduction checks are performed by switching the pacing mode from VDD to VVI with a lower rate of 40 bpm. If VS events occur at rate above 40 bpm, atrial sensing is turned off and the device remains in VVI mode. This explains the lack of atrial markers and only VS markers at rest (Figure 1B). The tracing recorded during exercise with a smartwatch shows sinus tachycardia and spontaneous QRS complexes (Figure 1C). The parameters were left unchanged.
Patient 2: Congenital AV block with escape rhythm
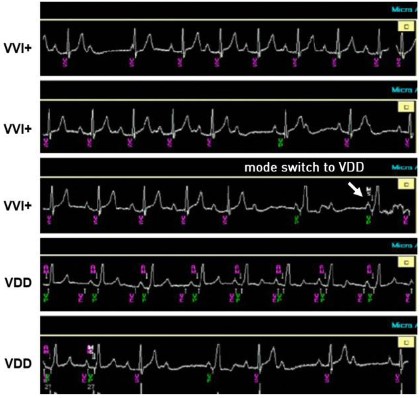
Patient 2 was a 22-year-old female with congenital AV block and an escape rhythm of 50 bpm. She presented with episodes of lightheadedness corresponding to ventricular pauses. At device check three months after device implantation she was asymptomatic. As with Patient 1, the ventricular pacing burden was very low, consistent with the device functioning in VVI+ mode most of the time (AV conduction mode switch). The first tracing (Figure 2a) registered during device interrogation and an ECG registered at home using a smartwatch (Figure 2b) show 2:1 AV conduction with a ventricular rate >40 bpm. When the VVI+ mode is activated, the MicraTM AV deactivates the atrial sensing function and strictly looks at the intrinsic ventricular rate to assess whether to switch back to VDD. Therefore, as long as the intrinsic ventricular rate is above 40 bpm, no ventricular pacing will be delivered irrespective of AV synchrony, including in cases of complete AV block with escape rhythms above this rate threshold (Figure 2c, during consultation) or 2:1 AV conduction at atrial rates exceeding 80 bpm, for example. Figure 3 shows a mode switch from VVI+ to VDD mode during the consultation. When in VVI+ mode, the device switches back to VDD mode when 2 beats out of a rolling window of 4 are paced at the set lower rate (40 bpm). The first check for AV conduction then occurs after 1 minute. This interval is doubled until a maximum time of 8 hours in the absence of an intrinsic ventricular rate above 40 bpm. In this patient, we have switched off the AV Conduction Mode Switch to preserve AV synchrony.
Patient 3: Complete AV block with high percentage of AV synchrony
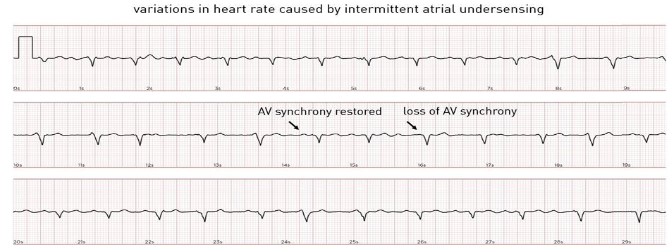
Patient 3 was a 28-year-old female with congenital AV block in whom an epicardial pacemaker had previously been implanted. She was then implanted with a MicraTM AV due to epicardial lead dysfunction. Three months after implantation she remained asymptomatic and underwent routine device interrogation. We observed a high amount of AV synchrony (80%, defined as the ratio of atrial mechanically [AM] sensed-VP to total VP percentage). The A4 amplitude trend suggests that signals in the A4 window have been above the minimal auto A4 threshold. During interrogation, AV synchrony indeed seems to be preserved during most cycles with an AM marker following the P-waves, but varied with physical activity and posture. A modest exercise test was performed during interrogation by performing arm movements (Figure 4). AV synchrony is initially well maintained. During exercise, the A3 and A4 signals merge, resulting in an “A7” signal. The A3 auto threshold function had been turned off during the pre-discharge interrogation to preserve AV synchrony during sinus acceleration with consistent tracking in the A3 window and no sensing and tracking of A3 signals at rest and during exercise. The fixed-A3 threshold was lowered sufficiently to avoid A4 undersensing when A3 and A4 are merged but kept high enough to avoid A3 oversensing. As the sinus rate increases, certain atrial events are undersensed (intermittent sensing of the A7 signal in the A3 window). The device incorporates a rate smoothing feature to maintain AV synchrony and to deliver ventricular pacing at a programmable rate smoothed interval during intermittent A4 undersensing. When a P-wave is undersensed, the device does not drop to the lower programmed rate but paces at a programmable rate smoothing interval. Although this algorithm prevents sudden rate drops, the heart rate can still become irregular with considerable variations in cycle lengths. Figure 5 shows a smartwatch ECG with slowing down of the heart rate during P-wave undersensing and speeding up during correct sensing. At higher heart rates (>105 bpm), the device switches to VVIR mode, therefore AV synchrony is lost. However, the heart rate stabilizes as cycle length variations due to intermittent atrial undersensing are no longer introduced. The patient’s rhythm was considered AV synchronous considering the high percentage of AM-VP, without signs of oversensing to suggest that this percentage was overestimated. Since the patient was asymptomatic at rest and during exercise, the programming was not changed.
Patients 4-6: Complete AV block with low percentage of AV synchrony
Patients 4-6 were aged 25, 21, and 26, and were each implanted with a MicraTM AV because of congenital AV block, AV block after aortic root surgery, and AV block with syncope, respectively. At three months, they shared a similar profile during device interrogation: all had sinus atrial activity but were dependent in the ventricle (100% VP) and their percentage of AV synchrony was low (45%, 33%, and 49%). However, each had different reasons for this low percentage:
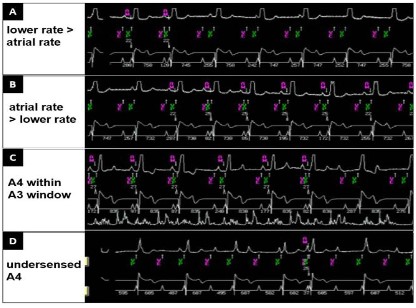
1. Lower rate set too high. The programming of a lower rate at 60 bpm in a patient with sinus rates between 55 and 65 bpm resulted in loss of AV synchrony (Figure 6A). Reprogramming the lower rate to 50 bpm resulted in an increase in AV synchrony (Figure 6B).
2. A3 window too long. In all three patients, shortening the A3 window improved issues with undersensing of atrial activity (A4). Figure 6C shows AV synchrony followed by under sensing of atrial activity. The A4 signal is falling in the A3 sensing window, preventing appropriate sensing of the A4 by the device and resulting in lack of AV synchrony. In this specific patient, we shortened the A3 window end interval to 750 ms (programmed Min Auto A3 Window End value to 700 ms, programmed Max Auto A3 Window End to 800 ms) to allow the device to detect the A4 signal.
3. A4 threshold too high. In two patients, we had to adjust the sensitivity threshold for the A4 signal. An appropriate A4 threshold is below the A4 signal but above noise. Figure 6D shows intermittent atrial undersensing (A4 occurs after the A3 window but remains undetected). In this specific patient, the amplitude of the signal in the A4 window was relatively stable but intermittently fell below the programmed minimal Auto A4 Threshold, explaining the absence of appropriate atrial tracking. We adjusted the A4 threshold (lowered it to 0.8 m/s2) and adequate sensing of A4 immediately improved. In the second patient, we had to modify the atrial sensing vector to find a larger A4 signal. The signal amplitude in the A4 window remained below the minimal Auto A4 Threshold despite it being optimized (set to 0.8 m/s2). With the maximum combination of vectors 1+2+3, the A4 signal amplitude increased resulting in restoration of AV synchrony.
In these 3 patients, reprogramming increased the percentage of AV synchrony over the “recommended” threshold of 70%. However, the percentage of atrial tracking remained imperfect due to intermittent A4 undersensing at rest, which worsened with activity.
Patient 7: Programming to VVIR mode when AV synchrony remains low
Patient 7 was a 24-year-old with a myopathy, high body mass index, and symptomatic complete AV block who received a MicraTM AV without complication. During the pre-discharge interrogation, the patient had a sinus atrial rate at 85 bpm at rest. After three months, an attempt to optimize AV synchrony was performed with shortening of the A3 window, fixing of the A3 threshold, and a search for a better vector.
Despite these efforts, we could not achieve an acceptable AV synchrony percentage. We ultimately reprogrammed the device to VVIR. The patient has remained asymptomatic.
Discussion
The cases presented show that the complexity of AV leadless pacemaker programming and the need for optimization during follow-up to achieve higher levels of AV synchrony must be acknowledged. The seven patients we describe were selected to illustrate troubleshooting strategies that an implanter should be familiar with to adequately manage young patients with leadless VDD pacemakers. Reprogramming and optimizing the MicraTM AV system can differ markedly from that of conventional dual chamber pacemakers with regards to atrial sensitivity, refractory periods, exercise behavior, and algorithms aiming to decrease the ventricular pacing burden.
The first two patients had ventricular rates above 40 bpm, highlighting the pros and cons of the AV Conduction Mode Switch algorithm (VVI+ mode), which is designed to prevent unnecessary right ventricular pacing. The first patient had a normal ECG but experienced paroxysmal high degree AV block. By reducing the ventricular pacing burden but assuring pacing support during episodes of AV block, VVI+ mode can save battery life and preserve physiological ventricular activation. In contrast, in the second patient with congenital AV block, an escape rhythm at 50 bpm, and normal chronotropic function, the VVI+ mode decreased the ventricular pacing burden at the expense of AV synchrony. Recognizing that the two proposed programming options have opposing effects is important to individualizing treatments, as one must balance the benefits of maximizing AV synchrony with the risks of iatrogenic interventricular/intra-ventricular asynchrony. The effects of the latter on long-term endpoints such as incident atrial fibrillation and heart failure are unknown.
For the five other patients with complete AV block, underlying sinus activity, and a lack of stable escape rhythms, a priority of reprogramming was to maximize AV synchrony. As previously explained, the device is not designed to track atrial rates above 105-110 bpm. As the sinus rate increases, the device is constrained by blanking and refractory periods designed to not detect A1, A2, or A3 signals. At higher heart rates, the pacemaker switches to VVIR modes thereby losing atrial synchronous function. Therefore, in young and physically active patients, there is appropriately little interest in implanting a leadless AV pacemaker over a standard leadless VR pacemaker to improve exercise capacity or to correct symptoms during exercise. The main goal of programming is instead to achieve AV synchrony at rest or during moderate exercise. In our experience, even with programming optimization before hospital discharge after implant, achieving satisfactory AV synchrony in patients with complete heart block requires further adjustment of atrial sensing parameters during outpatient follow-up.
Current leadless pacemakers do not have a memory to allow for automatic recording of EGM tracings of tachycardia or periods of loss of AV synchrony. For this reason, we equip our patients who have MicraTM AV devices with smartwatches capable of recording ECGs and ask them to perform a recording at least once a week, both in standing and supine positions. This allows for earlier recognition and correction of atrial sensing problems. In our patients with leadless pacemakers, at discharge and at three months, the most common programming adjustments required are as follows:
1. Lowering the A4 threshold (including lowering from that selected immediately after the implant procedure), turning the auto-sensing algorithm off.
2. Shortening of A3 detection window (shortening of min and max A3 window end compared to nominal settings), without programming Min A3 End <650 ms.
3. The A3 Threshold was systematically fixed (Auto A3 Threshold Off) to detect fused A3 and A4 signals that occurred during exercise (A3 Threshold 1.0–1.5 m/s2 above the largest isolated A3 signal amplitude observed).
4. The mechanical atrial sensing vector was changed in one patient due to atrial undersensing.
In Patient 7, the quality of the atrial signal was too poor, regardless of the programmed detection vector, to adequately detect atrial contraction and allow operation in VDD mode. This patient suffered from severe obesity and echocardiography suggested abnormal atrial contraction. In this patient, we ultimately opted to program the device to VVIR mode, which fortunately was not associated with any clinical symptoms. Further studies will be paramount for establishing predictors of AV synchrony with VDD leadless pacemakers in young patients. In the remaining patients, even if the degree of AV synchronization could be improved with the different settings proposed, atrial sensing remained imperfect with intermittent loss of detection that worsened by changes in heart rate, patient movement and atrial or ventricular arrhythmias. Although it is possible to program frequency smoothing to avoid sudden drops in frequency, interrogation of the MicraTM AV commonly found EGMs with adequate AV synchronization alternating with periods without adequate atrial detection. This resulted in cycle length variations that were generally not noticed by the patient but resulted in sometimes marked rate irregularity on EGMs and on frequency curves.
Conclusion
Programming changes to atrial sensing parameters are usually required to optimize atrial tracking and to maximize AV synchrony in MicraTM AV pacemakers. As leadless pacemaker implants become more common, studies comparing conventional with leadless systems and perhaps also leadless AV with leadless VVI systems will be required to inform the often difficult process of device selection in young patients.
References
- Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014; 35: 1186-1194.
- Roberts PR, Clementy N, Al Samadi F, Garweg C, Martinez-Sande JL, et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm. 2017; 14: 1375-1379.
- El-Chami MF, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande JL, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018; 15: 1800-1807.
- Garweg C, Splett V, Sheldon TJ, Chinitz L, Ritter P, et al. Behavior of leadless AV synchronous pacing during atrial arrhythmias and stability of the atrial signals over time-Results of the MARVEL Evolve subanalysis. Pacing Clin Electrophysiol. 2019; 42: 381-387.
- Strik M, Nicolas C, Mondoly P, Eschalier R, Ramirez FD, et al. Implantation of a leadless pacemaker in young adults. J Cardiovasc Electrophysiol. 2022.