Open Access, Volume 9
Severe iron-deficiency anaemia in a patient with heart failure: A case report and review of the literature
Ioana Bianca Haliga1; Alice Colescu1; Alexandra Condurat1; Bianca Codrina Morărașu1,2; Manuela Ursaru1,3; Elena Corina Andriescu1,4; Ludmila Știrbu5; Raluca Ecaterina Haliga1,2*
1University of Medicine and Pharmacy “Gr.T.Popa Iasi”, Faculty of Medicine, Romania.
2Department of Internal Medicine, “St. Spiridon” Emergency Clinical Hospital, Iasi, Romania.
3Department of Radiology, “St. Spiridon” Emergency Clinical Hospital, Iasi, Romania.
4Department of Morphopathology, “St. Spiridon” Emergency Clinical Hospital, Iasi, Romania.
5Department of Surgery, “St. Spiridon” Emergency Clinical Hospital, Iasi, Romania.
Raluca Ecaterina Haliga
University of Medicine and Pharmacy “Gr.T.Popa Iasi”, Faculty of Medicine, Romania.
Email: raluca.haliga@umfiasi.ro
Received : February 01, 2023,
Accepted : February 24, 2023
Published : February 28, 2023,
Archived : www.jclinmedcasereports.com
Abstract
Background: Anaemia of different etiologies, but also iron deficiency without anaemia, worse symptoms and prognosis of patients with heart failure or coronary artery disease. Development of anemia in patients with chronic heart failure is multifactorial, one of most important mechanism being chronic inflammatory activation that impairs iron metabolism. However, other possible causes of anaemia can coexist and investigations should be done to find them.
Case: We present the case of a female patient with chronic heart failure and coronary artery disease, whose clinical picture and evolution were progressively aggravated by a severe iron-deficient anaemia. Besides heart failure-related cause, other possible origins of anaemia have been searched. Colonoscopy revealed a source of occult bleedings, a cecum tumor. Intravenous iron treatment improved the general status and clinical evolution of the patient, allowing surgical treatment of the tumor.
Conclusion: In patients with chronic heart failure, extracardiac causes of anaemia should be carefully investigated and treated, when found. According to guidelines, the most efficient treatment for iron deficiency associated with heart failure is intravenous iron treatment.
Keywords: Anaemia; Iron-deficiency; Heart failure; Coronary artery disease; Cecal neoplasm; Ferric carboxymaltose.
Copy right Statement: Content published in the journal follows Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). © Haliga RE (2023)
Journal: Open Journal of Clinical and Medical Case Reports is an international, open access, peer reviewed Journal mainly focused exclusively on the medical and clinical case reports.
Citation: Haliga IB, Colescu A, Condurat A, Morărașu BC, Haliga RE, et al. Severe iron-deficiency anaemia in a patient with heart failure: A case report and review of the literature. Open J Clin Med Case Rep. 2023; 1987.
Introduction
Anaemia is one of the most common blood disorders, affecting about one third of the world population [1]. Chronic, occult blood loss is one of the most common etiologies of anaemia, leading to iron deficiency (ID) anaemia [2]. Chronic Heart Failure (CHF) is the end stage of multiple cardiac disorders, associated with different short-term and long-term consequences [3]. The evolution of CHF can be worsened by many factors, such as anaemia, infections, fever, or arrhythmias. In the context of CHF, ID and ID anaemia may be caused either by reduced iron intake, increased loss, decreased absorption (gut congestion) or by chronic inflammatory activation that impairs iron metabolism [2]. Even if CHF is present, extracardiac causes of anaemia should be sought and treated. Moreover, even in the absence of anemia, ID can be present in approximately half of patients with CHF [4]. This is associated with impaired functional capacity, skeletal muscle dysfunction and frailty, worsening symptoms and prognosis of patients with CHF. Different studies demonstrated that both anaemic and non-anaemic ID patients with CHF have recurrent hospitalizations and higher cardiovascular and all-cause mortality [5].
We present the case of a female patient with CHF and ischemic heart disease, whose evolution was aggravated by a severe anaemia. Work-up and diagnosis was challenging, as well as establishing the optimal treatment and balancing the patient’s status.
Case Report
Presentation: A 75-year-old woman presented to Emergency Department (ED) in June 2022 for resting shortness of breath, fatigue and angina-type chest pain. The symptoms got progressively worse a few days prior to presentation. Blood tests revealed severe normocytic, normochromic iron-deficient anemia (Hb 5.7 g/dl, serum iron 17 mg/dl and ferritin 16 ng/ml). An urgent upper digestive endoscopy was performed ruling out an active or recent bleeding, two gastric hyperplasic Paris 1s lesions being identified.
History: Past medical history consists of type 2 diabetes mellitus (2010), arterial hypertension, inferior myocardial infarction, with subsequent percutaneous transluminal coronary angioplasty (PTCA) and drug-eluting stent (DES) implantation on right coronary artery (2015) and chronic heart failure. Another PTCA with DES has been performed 30 days ago for new onset atrial fibrillation and unstable angina. At that time, a 75% atherosclerotic stenosis of the circumflex coronary artery was found and the hemoglobin (Hb) level was 9 g/dl. Current medication included triple antithrombotic therapy (Aspirin, Clopidogrel, Rivaroxaban), statin, beta-blocker, diuretic, nitrate, angiotensin-converting-enzyme inhibitor (ACEI), and insulin. Oral iron therapy for anemia was also prescribed, although the cause was not established.
Examination: On arrival in our Internal Medicine Department, the patient was conscious and oriented to time, place and person, with moderately altered general status. Clinical examination revealed pale skin and mucosa, arrhythmic heart sounds with no added murmurs, mild bilateral lower limb pitting oedema, abdominal distention, painful on palpation in the epigastric and right hypochondrium area.
Investigations: Further blood tests revealed mildly increased creatinine (1.37 mg/dl), with glomerular filtration rate (GFR) 50 ml/min/1.73 m2 (CKD-EPI), bone marrow hyporegeneration (reticulocytes count 30,000/mm3), increased N-terminal brain natriuretic peptide (NT-proBNP) of 5186 ng/ml (as a marker of heart failure), unsatisfactory glycemic control with glycated Hb 8.7%. Out of the determined tumoral markers, the carcinoembryonic antigen (CEA) was increased by approximately six folds (29,4 ng/ml). Peripheral blood smear showed erythrocytes anisocytosis and anisochromia, but also hypersegmented granulocytes. B12 vitamin and folic acid were within normal range. Fecal occult blood test could not be determined.
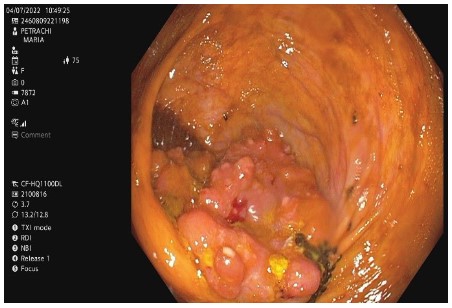
ECG revealed atrial fibrillation with ventricular rate of 80 bpm and anterolateral ischemic changes. Cardiac enzymes were within normal range. The echocardiography indicated hypertensive heart disease, with left ventricular (LV) hypertrophy and diastolic dysfunction, mild left and right atrial enlargement, mild functional mitral and tricuspid regurgitation, systolic LV dysfunction with moderate reduced ejection fraction (EF) 42%, aortic atheromatosis. The thorax-abdomen and pelvic computer tomography (CT) with intravenous and digestive contrast revealed thrombi in the left atrium, mild bilateral pleural effusion, and small hiatus hernia. CT could not identify a lesion suggestive for malignancy, considering also the unsatisfactory bowel preparation. We proceeded the investigations with an inpatient colonoscopy. It revealed a 4-5 cm cauliflower-shaped tumoral mass in the cecum, adjacent to the ileocecal valve (Figure 1). The anatomopathological exam showed papillary and tubular structures with high-grade epithelial dysplasia.
Management: During hospitalization, we adjusted the treatment, antithrombotic therapy was continued with low-molecular-weight heparin and Clopidogrel. The patient also received two units of packed RBCs and intravenous (i.v.) iron therapy (ferric carboxymaltose - FCM), with symptom improvement and progressive increase in Hb up to 12 g/dl. The patient was then transferred to the Surgical Department. Right hemicolectomy for tumoral resection within safety limits, with latero-lateral ileo-transverse anastomosis were performed. The intervention was difficult and prolonged due to surgical history of the patient (open cholecystectomy in 2005, followed by postoperative eventration, cured with nylon net and consecutive abdominal surgical scars).
Anatomopathological exam of the tumoral mass revealed ulcerated low-grade NOS (Not Otherwise Specified) colon adenocarcinoma (G1), invading thickness of submucosa and muscularis, localised up to the level of subserosa, without affecting serosa (Figure 2).
Outcome: The patient was admitted to the Intensive Care Unit for seven days after surgical intervention, with difficult evolution due to uncontrolled glycemic values, hemodynamic instability and renal failure. After inotropic support and specific treatment were applied, there was a good clinical response. On discharge, the patient was referred to Oncology Department, for further specific monitoring and treatment. Currently, she is in a good clinical condition, without anaemia, continuing the cardiac treatment and under oncological monitoring, without chemotherapy initiation.
Discussion
In patients with CHF, development of anemia is multifactorial. Renal dysfunction and defective erythropoiesis, as well as neurohormonal and pro-inflammatory citokines (Tumor Necrosis Factor-α (TNFα) and interleukin-6 (IL-6)) activation are involved in CHF and can contribute to ID. Hepcidin, a protein released from the liver by IL-6, can cause ID in CHF through different pathways, including hepcidin-induced failure of iron absorption from the gut, reduced re-absorption of recycled iron and trapping of iron in the macrophages and hepatocytes stores [3,5-7].
Our patient, known with chronic and complex cardiac pathology, progressively developed an ID anemic syndrome. Severe anemia worsened the clinical manifestations of heart failure (dyspnea), associated with increased value of biological parameter of HF (NT-proBNP). One month ago, our patient received recommendation for triple antithrombotic therapy in the Cardiology Department, after DES coronary implantation and new-onset atrial fibrillation. Worsening anaemia in the context of new diagnosis of cecum tumor made us consider that the triple antithrombotic therapy induced the tumoral bleeding. On one hand, antithrombotic therapy was mandatory for patient’s cardiac pathology, on the other hand, anaemia, by decreased RBC count and Hb value, reduces oxygen supply and worsens myocardial hypoxia, aggravating heart failure symptoms and myocardial ischemia. If present in CHF, anemia is associated with worsening symptoms, increased serum levels of natriuretic peptides, as markers of HF, recurrent hospitalization and higher mortality [8].
The most recent guideline for diagnostic and treatment of HF recommend that all patients with HF should be regularly screened for anaemia and ID [2]. When detected, doctors should promptly investigate the cause. Subsequently, we can initiate appropriate treatment which should improve symptoms and evolution. Our patient had moderate anaemia detected from previous hospitalisation, one month ago. During admission in our department, anaemia became more severe, prompting us to consider a gastrointestinal source of bleeding, probably on occult one, as there were no signs of exteriorisation. While upper digestive endoscopy did not find a source of bleeding, colonoscopy revealed the cecum tumor.
Randomized clinical trials have demonstrated that iron supplementation with i.v. FCM are indicated in patients with HF and left ventricle EF under 45%, for improvement of symptoms, exercise capacity and quality of life, also for reduction in recurrent CV or HF hospitalizations and related-death.9,10 Intravenous FCM should also be considered in patients with mildly reduced EF of left ventricle, recently hospitalized for worsening HF, as it was the situation in our patient [11,12].
In our patient, Hb level increased by packed RBCs transfusions and i.v. iron administration, as indicated by guidelines [2]. Thus, anaemia was corrected, and the patient's condition and symptoms of HF temporarily improved, allowing the surgical treatment. The diagnosis of cecum tumor and absence of extensions on CT exam (metastasis or lymphadenopathy) led to transfer of the patient to Surgical Department. The tumor was completely removed by surgery and thus eliminated the risk of bleeding determined by resuming of the antithrombotic treatment for cardiac pathology with vital risk.
Conclusion
The investigation of a patient with heart failure and anaemia is chalenging and all efforts should be done to find out if there is another cause of anaemia instead of the heart failure-related one. Also, as indicated by guidelines, correction of anaemia with blood transfusions, if indicated, but mainly with i.v. iron as FCM improve the symptoms and prognosis of patients with heart failure of different causes.
Acknowledgments: The authors are grateful to the patient for agreement of publication of the case.
Conflict of interest statement & funding: The authors have no conflicts of interest, funding or financial relationships to declare.
References
- Safiri S, Kolahi AA, Noori M, Nejadghaderi SA, Karamzad N, et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. J Hematol Oncol. 2021; 14: 185.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599-3726.
- Dalal J, Katekhaye V, Jain R. Effect of ferric carboxymaltose on hospitalization and mortality outcomes in chronic heart failure: A meta-analysis. Indian Heart J. 2017; 69: 736-741.
- Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001; 131: 676S-90S.
- Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, Haehling S, et al. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail. 2016; 18: 786-795.
- Silverberg DS, Wexler D, Schwartz D. Is Correction of Iron Deficiency a New Addition to the Treatment of the Heart Failure? Int J Mol Sci. 2015; 16: 14056-14074.
- van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011; 8: 485-493.
- Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, et al. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014; 174: 268-275.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, et al. CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015; 36: 657-668.
- van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Bohm M, et al. EFFECT-HF Investigators. Effect of ferric carboxy-maltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017; 136: 1374-1383.
- Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al. AFFIRM-AHF Investigators. Ferric carboxy-maltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020; 396: 1895-904.
- von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019; 7: 36-46.